DOWNLOAD ARTICLE
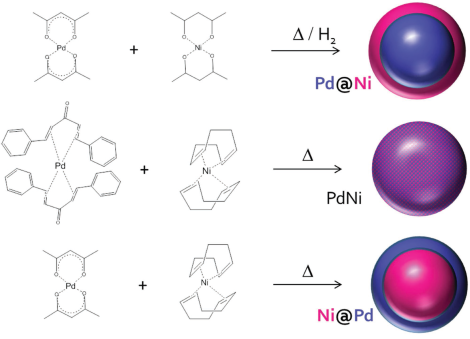
Abstract: The development of alkaline exchange membrane fuel cell (AEMFC) is limited by the sluggish reaction at the anode. Even precious group metals (PGMs) are not effective hydrogen oxidation reaction (HOR) electrocatalysts in alkaline medium. In this manuscript, the original synthesis of effective HOR
electrocatalysts for AEMFC is reported. Here, the limitations of using metalorganic precursors are described and their replacement with organometallic precursors is proposed. It is shown that completely different nanostructures can be synthesized by the organometallic route, resulting in the formation of NiPd nano-alloy or Ni@Pd core–shell nanoparticles, instead of Pd@ Ni. The presence of both Pd and Ni on the catalyst surface has a drastic effect on its HOR activity, due to a bifunctional electrocatalytic mechanism with hydrogen binding on Pd and OH binding on Ni. The highest activity is measured for NiPd nano-alloy, whose specific activity reaches 104 mA mg Pd -1 and 1.38 mA cm Pd -2 at 0.1 V versus reversible hydrogen electrode at 298 K. These are the highest values reported so far for an NiPd catalyst. By design, the synthetic approach is generic and can be applied to any pair of metals, either PGM or other transition metals, to synthesize alloyed or core–shell electrocatalysts.
