Watch The new Publication page of our new articles!
ACS applied Energy Materials: 2018, 1 (9), pp 4678–4685
Link
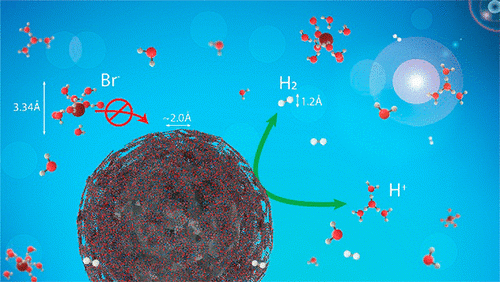
Abstract:Hydrogen–bromine redox-flow battery (RFB) technology offers the most economic storage solution and is considered most promising for a sustainable electricity storage solution due to its fast kinetics, highly reversible reactions, and low chemical costs. The main bottleneck of conventional electrodes is the rapid fading of the hydrogen catalyst performance in the highly corrosive environment. Here, we show that a simple coating of the catalyst can effectively protect the catalyst surface from corrosion in concentrated HBr and maintain a high catalytic activity. We polymerize dopamine on the surface of the catalysts and apply a gentle annealing step to obtain a few nanometers thin conformal polydopamine layer, which acts as a semipermeable barrier that effectively blocks Br. The catalytic activity was measured on a glassy carbon rotating disc electrode after dipping in 3 M HBr at 40 °C to accelerate the corrosion. The unprotected catalyst is irreversibly poisoned after 30 min (50% activity loss), while the hydrogen oxidation reaction activity of the protected catalyst remains high, even after dipping the catalyst layer in concentrated HBr for hours, with almost unchanged hydrogen diffusion constant. In principle, the polydopamine coating technique is compatible with all existing catalysts, as the polymerization involves only a room temperature step in a buffered aqueous solution and the coating displays an excellent adhesion to any substrate.
Keywords:
coating; corrosion; electrocatalysis; nanoparticles; polydopamine; Redox flow batteries; regenerative fuel cell
